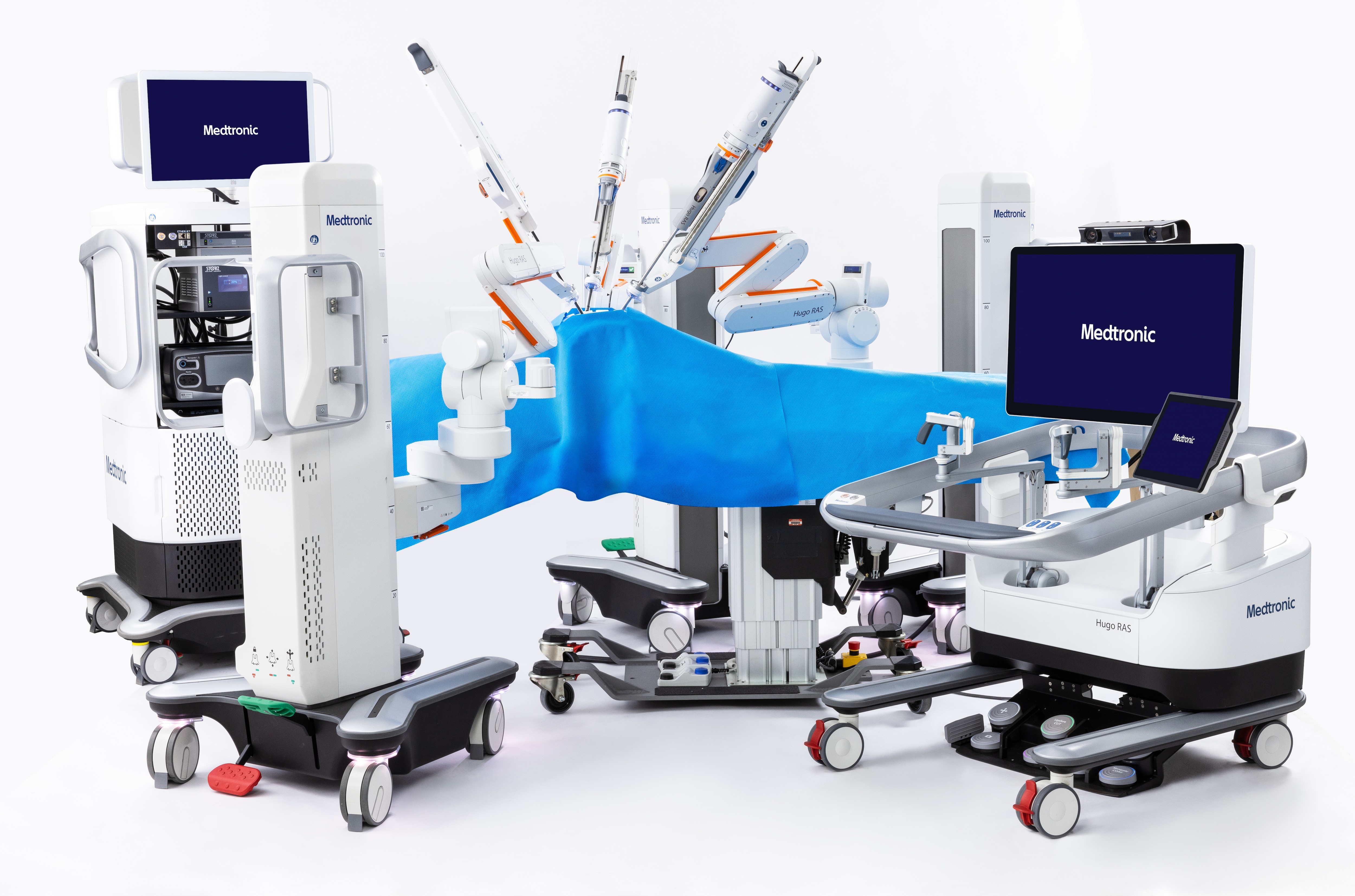
Introducing Hugo™
Find out how Hugo™ RAS system can make more possible for hospitals, surgeons and patients.
You know better than anyone, every surgery impacts a life. You’re in it to make a difference. We are too. We believe that when you combine your expertise and dedication with the right partner, extraordinary things can happen. Let’s make a difference, together.
Crafting a holistic surgical program takes a partner who understands your needs from a 360-degree perspective. Our dedicated team works with you to ensure optimized use of the Hugo™ robotic-assisted surgery system. And we support you through thoughtful planning to help build a sustainable holistic surgical program.
You’re committed to continuous improvement. So are we. That’s why we’ve made the Touch Surgery™ ecosystem an integral part of our robotic platform. Our digital ecosystem gives you access to the data you need to consistently improve performance3 and fine-tune your surgical programs. Available only with the Hugo RAS system in Canada.
Ready to see the Hugo™ RAS system in action?
You just clicked a link to go to another website. If you continue, you will leave this site and go to a site run by someone else.
Medtronic Canada does not review or control the content on the other website, and is not responsible for any business dealings or transactions you have there. Your use of the other site is subject to the terms of use and privacy statement on that site.
It is possible that some of the products on the other site not be licensed for sale in Canada.