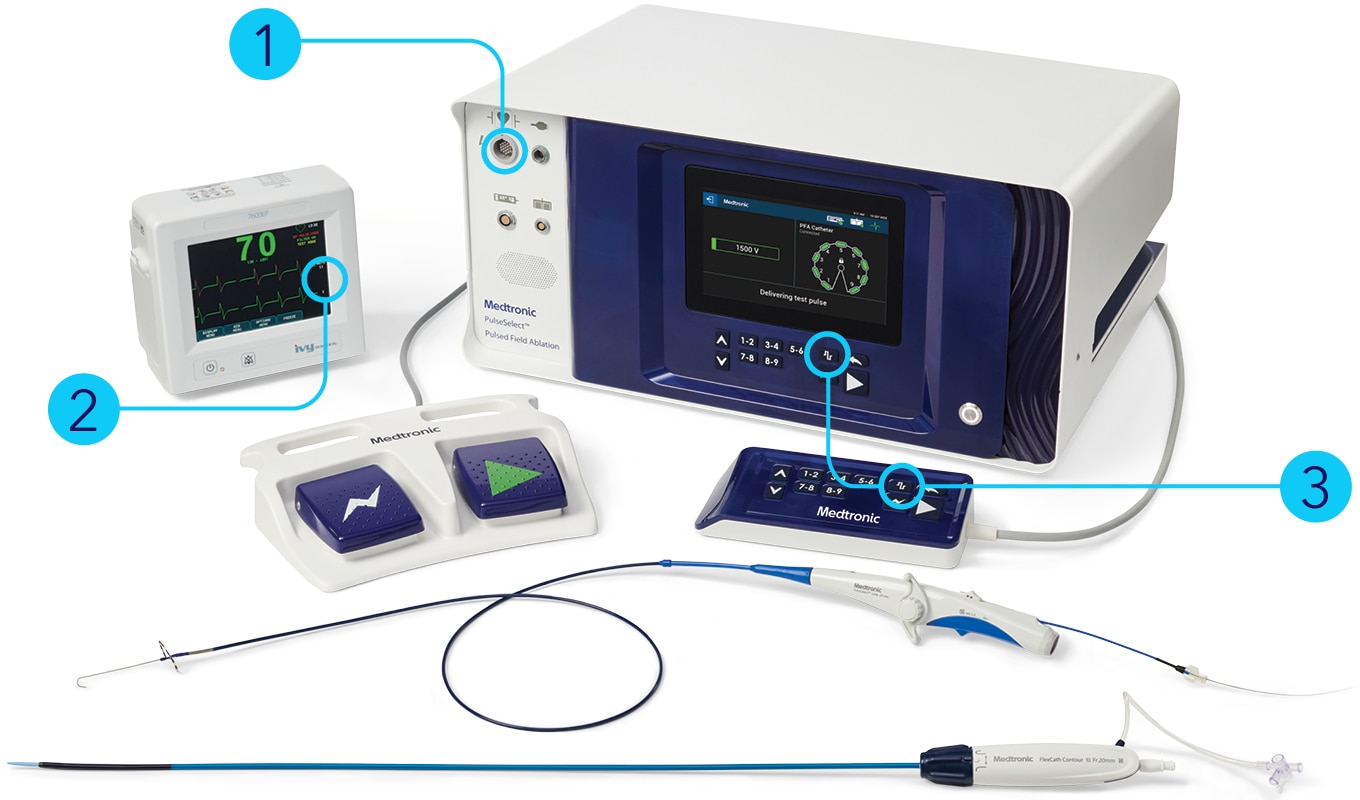
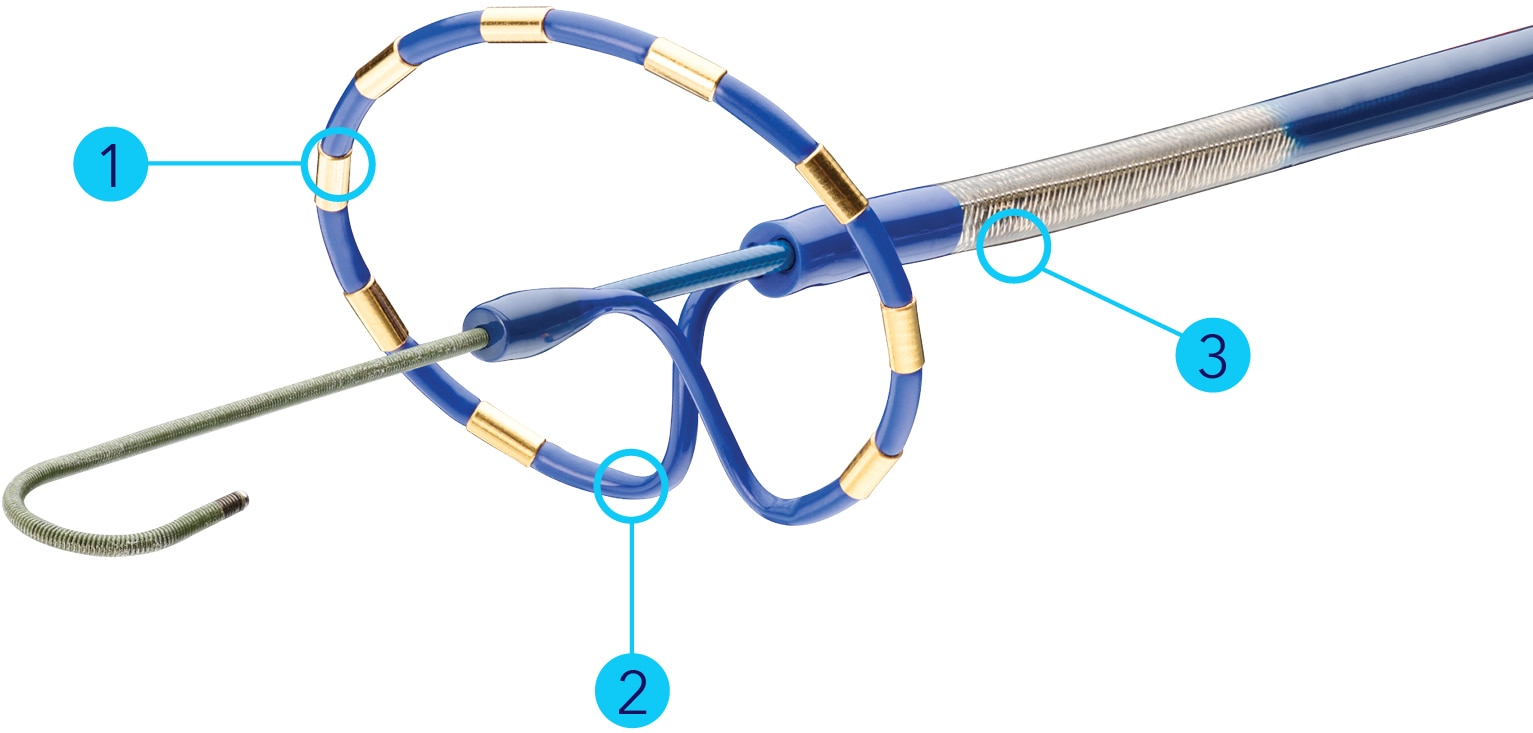
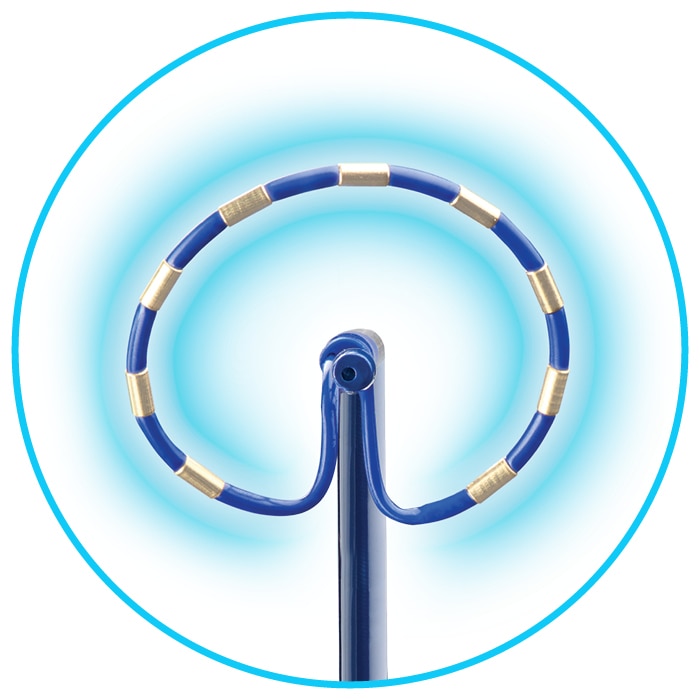
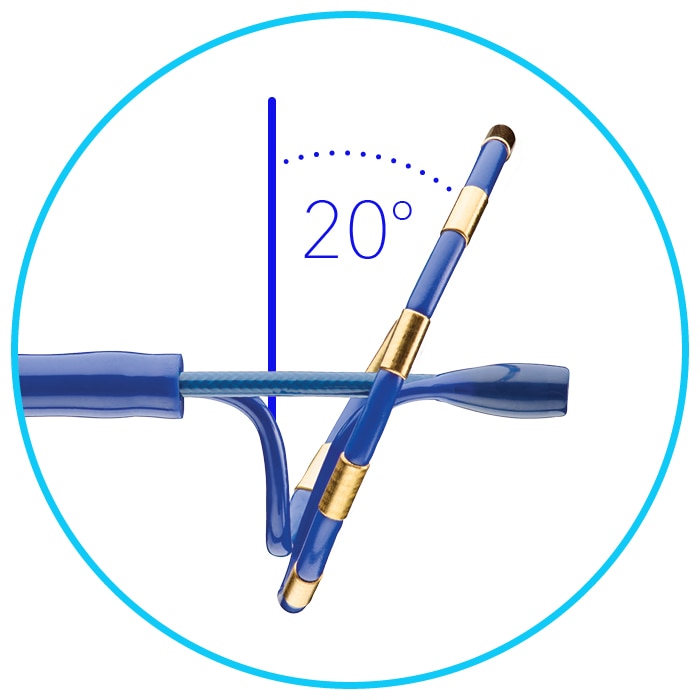
Indications (or intended use)
The PulseSelect™ pulsed field ablation (PFA) system is indicated for use in cardiac electrophysiological mapping (stimulation and recording) and for treatment of drug refractory, recurrent, symptomatic paroxysmal atrial fibrillation or persistent atrial fibrillation (episode duration less than one year).
Contraindications
The PulseSelect PFA loop catheter is contraindicated for use in patients with the following conditions:
- Active systemic infections
- A known sensitivity to Heparin
- Blood clotting abnormalities
The catheter is also contraindicated in conditions where the manipulation of the catheter within the heart would be unsafe, such as intracardiac mural thrombus.
The catheter is not recommended for use in patients who cannot undergo standard anticoagulation protocol for a left-sided cardiac procedure, or who have had a recent coagulopathy or embolic event.
Warnings and precautions
Ablation therapy hazards — To reduce the possibility of hazards associated with use of the PulseSelect PFA loop catheter:
- Use the catheter only in the recommended anatomical location.
- Maintain the catheter position during the ablation.
- Do not deliver energy near permanently implanted metallic objects.
- If coughing occurs, reposition the catheter more proximally and review sedation management.
- Ensure electrodes are not in contact with any metal during ablations (for example the guidewire).
- Maintain substantially circular array to ensure uniform field distribution.
- If the electrode array is deployed to deliver ablation energy, avoid continuing to move the slide control forward to prevent the guide wire lumen from coming too close to the electrode array.
Air entrapment — It is recommended that the array be captured while it is submerged to help reduce the possibility of air becoming entrapped around the electrode array during capture and catheter insertion.
Anticoagulation therapy — Administer appropriate levels of anticoagulation therapy for patients undergoing left-sided and transseptal cardiac procedures to minimize the risk of thromboembolic events. It is recommended to maintain therapeutic anticoagulation levels, per guidelines for patients undergoing cardiac ablation procedures.
Biohazard disposal — Discard all used catheters and sterile accessories in accordance with hospital procedures.
Catheter integrity — Use care to avoid damage to the catheter.
- Do not bend or kink the leading end of the catheter. Doing so could cause damage to the catheter lumen and make it unusable.
- Monitor the catheter throughout the procedure. If a flash is observed in the luer, replace the catheter immediately.
Do not resterilize — Do not resterilize this device for the purpose of reuse. Resterilization may compromise the structural integrity of the device or create a risk of contamination of the device that could result in patient injury, illness, or death.
Electrode-electrode contact — Avoid contact between electrodes. Contact between electrodes may create a short circuit.
Embolism risk — Introducing any catheter or sheath into the circulatory system entails the risk of air, gas, or thromboembolism, which can occlude vessels and lead to tissue infarction with serious consequences.
- Avoid unnecessary catheter exchanges to minimize sheath-related embolic events.
- Always advance and withdraw components slowly to minimize the vacuum created and the risk of air embolism.
- Aspirate and flush the sheath frequently to help minimize the potential for embolic events resulting from the introduction of air or clot formation within the sheath.
Fluoroscopy use during catheter placement — The use of fluoroscopy during catheter ablation procedures presents the potential for significant x-ray exposure to both patients and laboratory staff. Extensive exposure may result in acute radiation injury and increased risk for somatic and genetic effects. Only perform catheter ablation after giving adequate attention to the potential radiation exposure associated with the procedure, and taking steps to minimize this exposure. Give careful consideration before using the device in pregnant women.
For single use only — The PFA catheter is intended only to be used once for a single patient. Do not reuse, reprocess, or resterilize the PFA catheter. Reuse, reprocessing, or resterilization may compromise the structural integrity of the device or create a risk of contamination of the device that could result in patient injury, illness, or death.
Handling and placing catheters — Careful manipulation of the catheter is necessary to avoid cardiac damage, perforation, or tamponade.
- Do not use excessive force to advance, withdraw, or rotate the catheter, especially if resistance is encountered. Excessive force may lead to catheter damage and blood loss.
- Use imaging guidance during catheter advancement, manipulation, and placement.
- Vascular perforation is an inherent risk of catheter placement.
- Performing ablation with the PFA catheter array inside the sheath may result in damage to the array or the sheath and should be avoided.
- Performing steering manipulation with the PFA catheter array inside the sheath may result in damage to the catheter steering mechanism or the sheath and should be avoided.
- The PFA generator is capable of delivering significant energy. Do not touch the ablation electrodes of the PFA catheter while operating the generator.
- If the system is to be tested outside of the body, the electrode array must be immersed in saline solution in a plastic container. Never test PFA delivery in direct contact with skin.
Imaging for catheter placement — Use of imaging during catheter manipulation and placement is strongly advised. Manipulating the catheter without imaging may result in damage to cardiac and vascular structures.
Improper connection — Do not connect the PFA catheter to anything other than the PulseSelect PFA generator or use it to deliver non-PFA energy. This may cause catheter malfunction or patient injury.
Other devices, wires, or catheters — Avoid catheter entanglement with other devices, wires, or catheters, for example, intracardiac echo catheters. Failure to do so may increase the risk of entrapment of the array or damage to the array, which may affect retrieval of the device into the transseptal sheath.
Product literature — Do not operate the PFA system or connect a catheter to the generator before completely reading and understanding the product literature for the system.
Phrenic nerve injury — To reduce the potential for phrenic nerve injury, assess for proximity of the ablation catheter to the nerve using an appropriate technique such as pacing for local phrenic nerve capture or using the test pulse feature before ablation. Stop ablation immediately if phrenic nerve impairment is observed and assess for injury.
Required use environment — Cardiac ablation procedures should be performed only in a fully equipped electrophysiology laboratory.
Safety in magnetic resonance imaging (MRI) not evaluated — The PFA catheter has not been evaluated for safety and compatibility in the magnetic resonance (MR) environment. The safety of the PFA catheter in the MR environment is unknown (such as any heating, migration, or image artifact). Scanning a patient during the use of this device may result in patient injury.
Serious incident — If a serious incident related to the device occurs, immediately report the incident to Medtronic and the applicable competent authority or regulatory body.
Sheath and guide wire required — Do not attempt to advance or withdraw the catheter through the vasculature without the use of a sheath and guide wire, as it may result in damage to cardiac and vascular structures.
Sterile package inspection — Visually inspect the sterile packaging and catheter before use. If the sterile packaging or the catheter is damaged, do not use the catheter and contact your local Medtronic representative.
Storage and transport — There is no special handling, including no special storage or transport conditions, required for this device. Standard storage conditions are sufficient to safeguard the device. Store the device in the original packaging at room temperature in a dry place.
Use by date — Do not use the device after the “Use by” date on the package label.
Using PFA energy near implanted devices — Implanted devices, such as pacemakers and implantable cardioverter-defibrillators (ICDs), may be adversely affected by PFA energy.
- Keep external sources of pacing and defibrillation available during ablation.
- Program pacemaker sensing parameters to asynchronous pacing to ensure that PFA energy is not sensed as an intrinsic event.
- Deactivate ICD detection during the delivery of PFA energy.
- Perform complete implantable device testing before and after ablation.
- Monitor surface and intracardiac electrograms or vital signs during PFA energy delivery to assess for device interaction. Take appropriate action if any interaction is detected.
- Refer to the appropriate implantable device technical manual for additional information.
Potential adverse events or potential complications
Potential adverse events associated with cardiac catheter ablation procedures include, but are not limited to, the following conditions:
- Access site complications (such as, bruising, ecchymosis, arteriovenous fistula, hematoma, pseudoaneurysm)
- Anemia
- Arrhythmias, proarrhythmia (such as, atrial flutter, bradycardia, heart block, tachycardia)
- Bleeding, possibly requiring transfusion
- Bruising
- Cardiopulmonary arrest
- Perforation of the heart or other organs during transseptal puncture or other procedures
- Cardiac tamponade
- Catheter entrapment in cardiac structures requiring intervention
- Cerebrovascular accident such as stroke, transient ischemic attack (TIA)
- Chest discomfort, pain, or pressure
- Collateral damage to the conduction system or coronary vasculature
- Cough
- Death
- Embolism
- Esophageal damage (including atrial esophageal fistula)
- Hemoptysis
- Hypotension
- Hypertension
- Infections (such as, sepsis)
- Myocardial infarction or ischemia
- Nerve injury or nerve damage (for example phrenic nerve injury)
- Pericarditis or endocarditis
- Pericardial effusion
- Pneumothorax
- Pulmonary edema
- Pulmonary vein dissection
- Pulmonary vein stenosis
- Radiation injury or damage and late malignancy
- Skin laceration or puncture
- Sore throat
- Unintended complete or incomplete atrioventricular node (AV-Node) or sinus node block or damage
- Valvular insufficiency or damage.
Refer to the device technical manual for detailed information regarding the procedure, indications, contraindications, warnings, precautions, and potential complications/adverse events. For further information, please call Medtronic at 800-328-2518 and/or consult the Medtronic website at www.medtronic.com.
Caution: Federal law (United States) restricts these devices to sale by or on the order of a physician.
MAJ_85977