PF ablation systems
PulseSelect™ pulsed field ablation system
PF ablation systems
PulseSelect™ pulsed field ablation system
The PulseSelect™ pulsed field ablation (PFA) system enables both mapping and precise lesion delivery using biphasic waveform optimization.
Description
Setting a new standard in safety. Engineered for efficiency.
The PulseSelect™ PFA system is setting a new standard in safety for paroxysmal and persistent atrial fibrillation ablation, supported by 15 years of robust preclinical and clinical evidence. The PulseSelect™ PFA system offers a differentiated approach to pulsed field ablation that can be implemented across workflows, driving safe, effective, and efficient procedures.1
PulseSelect™ PFA system design and safety features
Unmatched safety1
Engineered with differentiated safety features from 15 years of PFA research and backed by one of the safest investigational device exemption (IDE) AF ablation trials to date.
Consistent efficiency
Rapid, effective pulmonary vein isolation (PVI)1 through consistent and predictable energy delivery and catheter maneuverability
Simplified adaptability2,3
Seamless transition to PFA with freedom to adapt to your preferred workflow
PulseSelect™ generator
- Automatic overcurrent detection for safe energy delivery
- R-wave gating for synchronization of energy delivery
- Test pulse for proximity detection to phrenic nerve
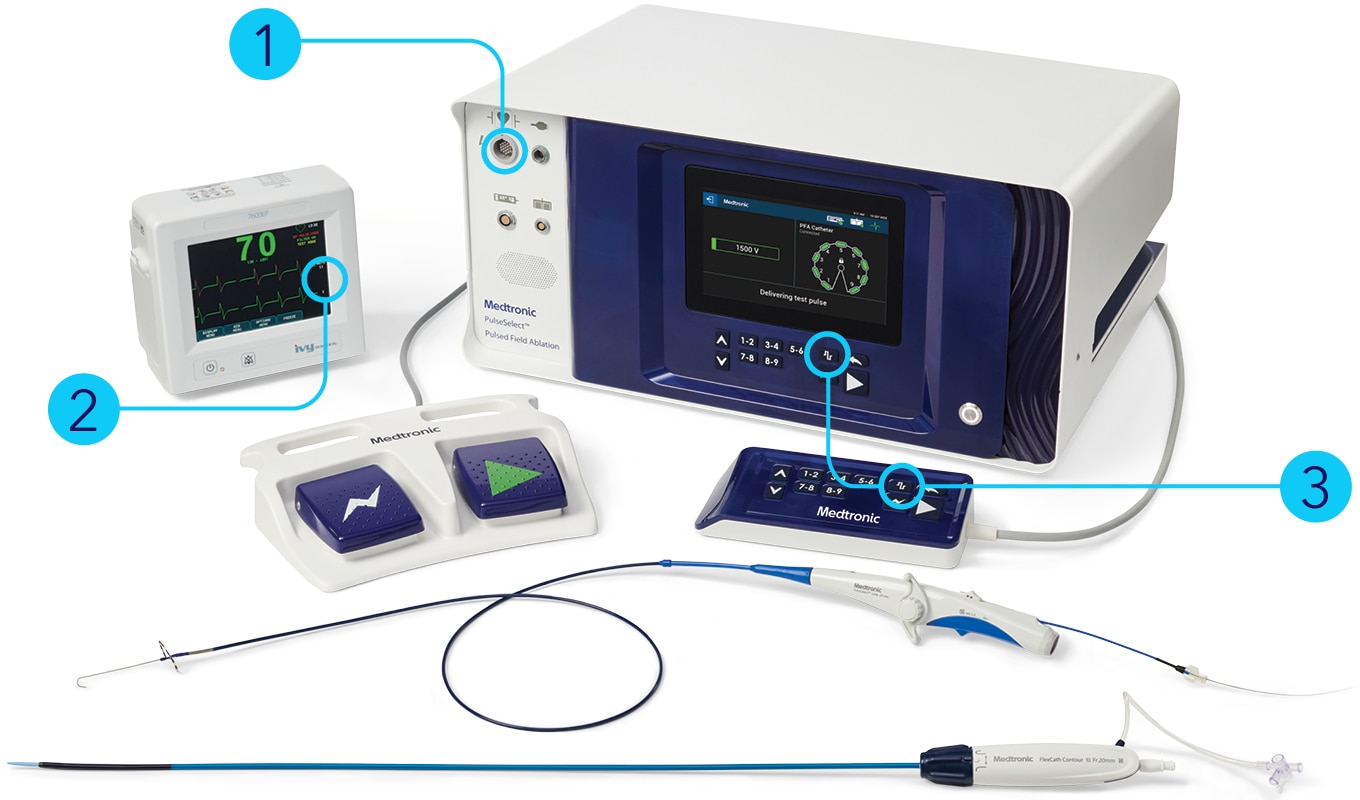
PulseSelect™ pulsed field ablation (PFA) system + FlexCath Contour™ steerable sheath
PulseSelect™ PFA catheter features
- 9 electrodes built to sense, ablate, and pace
- 25 mm diameter loop
- 9 Fr shaft with bidirectional steering
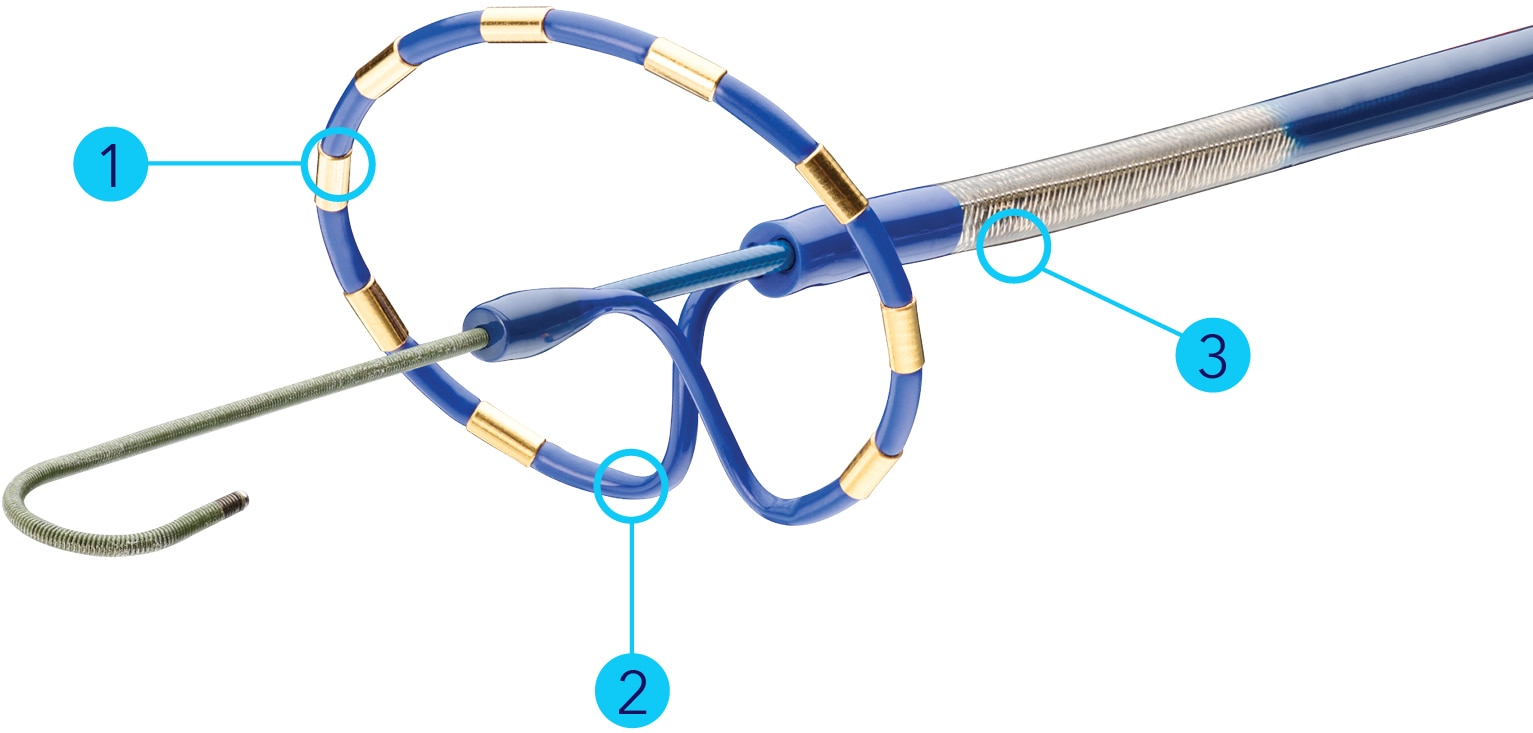
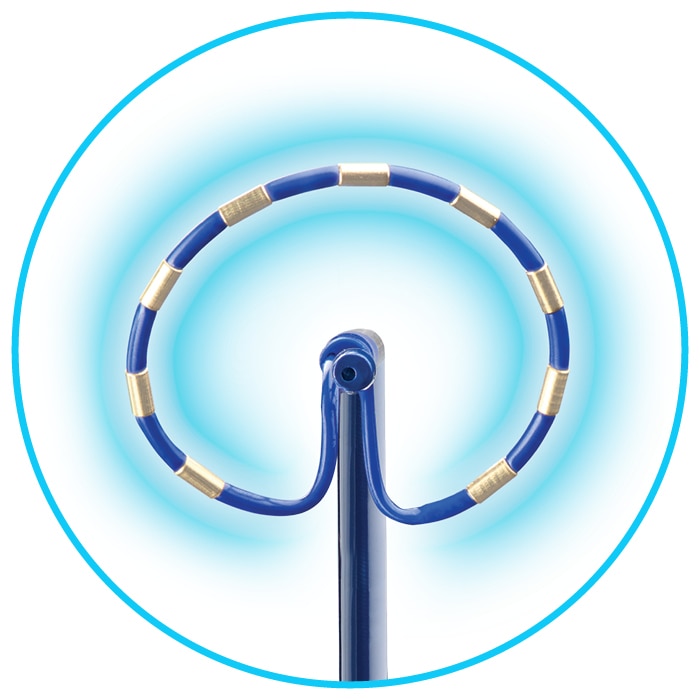
Fixed electrode spacing to produce a reliable field for predictable and consistent contiguous energy delivery
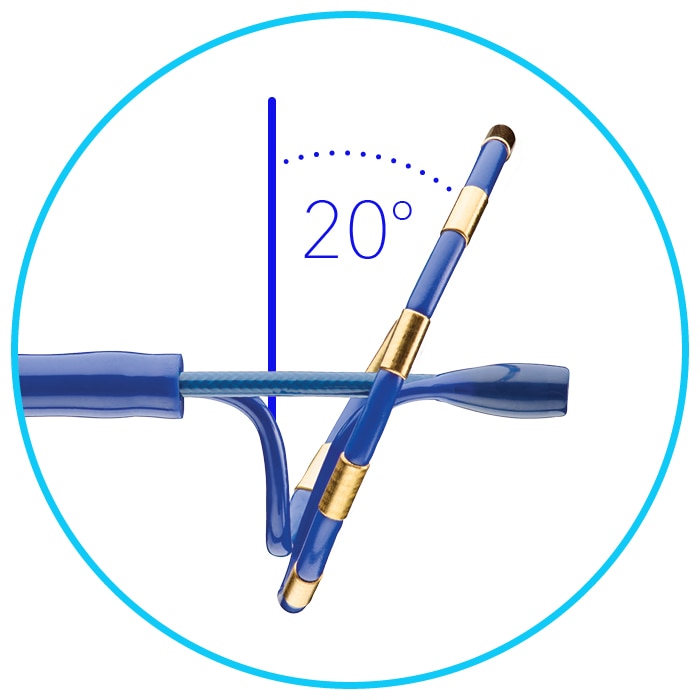
20-degree forward tilt to ensure more consistent uniform tissue contact
Trials
PULSED AF clinical trial1
PULSED AF evaluated the safety and effectiveness of the PulseSelect™ PFA system for the treatment of patients with paroxysmal and persistent atrial fibrillation and resulted in one of the lowest safety event rates of any AF ablation IDE trial to date.
0.7%
(primary safety event rate†)
70%
PAF
(freedom from AF/AFL/AT)
62%
PsAF
(freedom from AF/AFL/AT)
† Total of 13 adverse events measured; resulted in one cerebrovascular accident and one tamponade.
Ordering information
Resources
Related products
- Verma A, Haines DE, Boersma LV, et al. Pulsed field ablation for the treatment of atrial fibrillation: PULSED AF Pivotal Trial. Circulation. 2023;147(19):1422–1432.
- Based on internal test report (D01071310), Formal report for compatibility.
- Based on internal test report (D01071316), Characterization report with all additional image.





