| Item number guide |  |
Deep venous stents
Abre™ venous self-expanding stent system
Deep venous stents
Abre™ venous self-expanding stent system
The Abre™ venous self-expanding stent system is designed for the unique challenges of venous disease.
Description
The Abre™ venous self-expanding stent system offers easy deployment to let physicians focus on their patient and delivers demonstrated endurance to give patients freedom of movement.1,2
Features
Simplicity for you.
Durability for them.
Watch the Abre™ stent mechanism of action.
Indications
The Abre™ stent system is intended for use in the iliofemoral veins for the treatment of symptomatic venous outflow obstruction.
Clinical evidence
Clinically meaningful impact on quality of life through 36 months — in even the most complex patients.
36-month primary patency†,1:
- 81.6% overall
- 97.1% NIVL
- 76.5% aDVT
- 70.4% PT
Product details
Simplicity for you1,2
Hear it from the expert.
Easy deployment to let you focus on your patient.1,2

The Abre™ stent minimizes jumping and foreshortening, landing precisely where you need it.2
Rotating thumbwheel offers predictable placement and auditory feedback.2
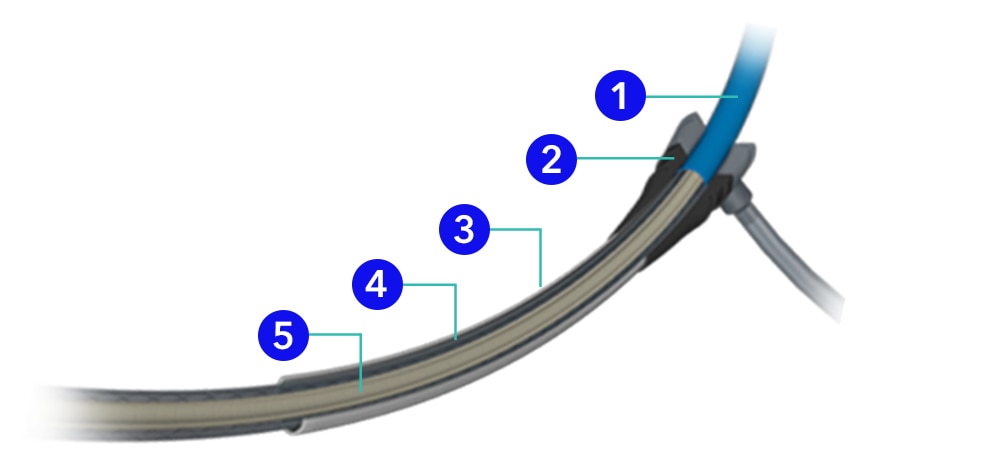
- Isolation sheath
- Hemostatic valve
- Introducer sheath
- Retractable sheath
- Inner shaft
Durability for them.1,2
Hear it from the expert.
Demonstrated endurance to give your patients freedom of movement.1,2
The nitinol Abre™ stent maintains lumen integrity and flow in diverse patients and anatomies.1 It ensures radial strength and crush resistance, without compromising flexibility.2
Unique technology:
- Open-cell design with three offset connection points
- Struts customized to each size
Consistent behavior across a broad range of diameters and lengths.2

Bench evidence shows long-term durability.2 Clinical evidence shows real-world dependability, even in challenging cases.1
- 0% fracture rate in clinical trial with 44% of stents extending below inguinal ligament into the CFV‡,1
- 0% migration rate in clinical trial1
Ordering information
| Stent diameter (mm) | Stent length (mm) | |||||
|---|---|---|---|---|---|---|
| 40 | 60 | 80 | 100 | 120 | 150 | |
| 10 | AB9U10040090 | AB9U10060090 | AB9U10080090 | AB9U10100090 | AB9U10120090 | AB9U10150090 |
| 12 | — | AB9U12060090 | AB9U12080090 | AB9U12100090 | AB9U12120090 | AB9U12150090 |
| 14 | — | AB9U14060090 | AB9U14080090 | AB9U14100090 | AB9U14120090 | AB9U14150090 |
| 16 | — | AB9U16060090 | AB9U16080090 | AB9U16100090 | AB9U16120090 | AB9U16150090 |
| 18 | — | AB9U18060090 | AB9U18080090 | AB9U18100090 | AB9U18120090 | AB9U18150090 |
| 20 | — | AB9U20060090 | AB9U20080090 | AB9U20100090 | AB9U20120090 | AB9U20150090 |
Related links
† Primary patency was defined as meeting all of the following: freedom from occlusion or restenosis ≥ 50% of the stented segment of the target lesion and freedom from clinically driven target lesion revascularization.
‡ Site and core lab data were used.
- Abre™ venous stent instructions for use.
- Test data on file at Medtronic. Report 10558227DOC_Rev A. Bench test results may not be indicative of clinical performance.