Interventional treatment for VCFs
Each year osteoporosis causes more than 750,000 vertebral compression fractures in the United States alone. Balloon kyphoplasty helps restore lost vertebral body height, stabilizes the fracture, and relieves pain — reducing reliance on opioid pain medications.1–4
VCF care pathway
A multispecialty expert panel used the RAND™/UCLA appropriateness method (RAM) to develop patient-specific guidelines for treating vertebral compression fractures.5

Kyphon™ balloon kyphoplasty
Balloon kyphoplasty (BKP) is a minimally-invasive procedure that uses inflatable bone tamps to reduce fractures and create a void for high-viscosity bone cement.
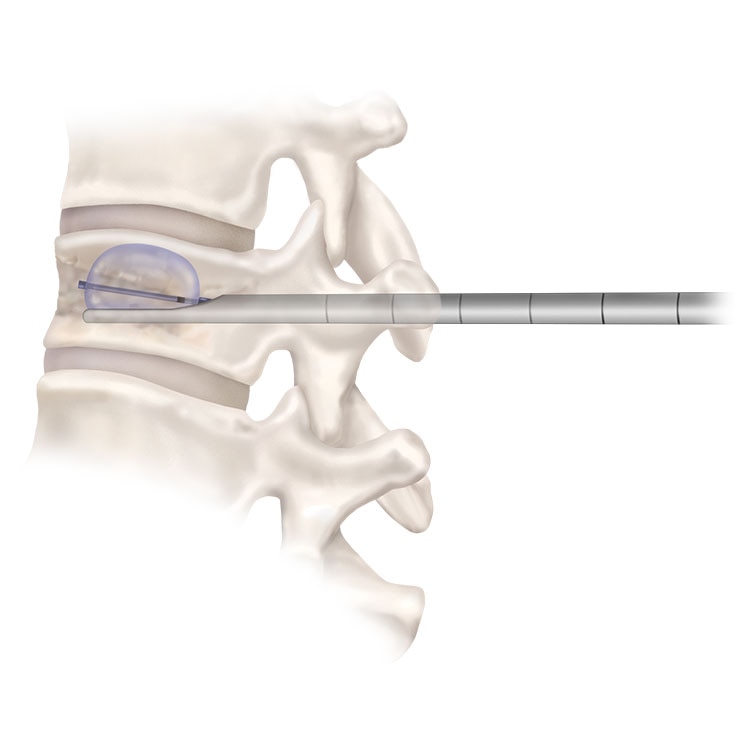
Vertebroplasty
Vertebroplasty is a minimally-invasive way to stabilize fractures by injecting bone cement into the fractured vertebra.
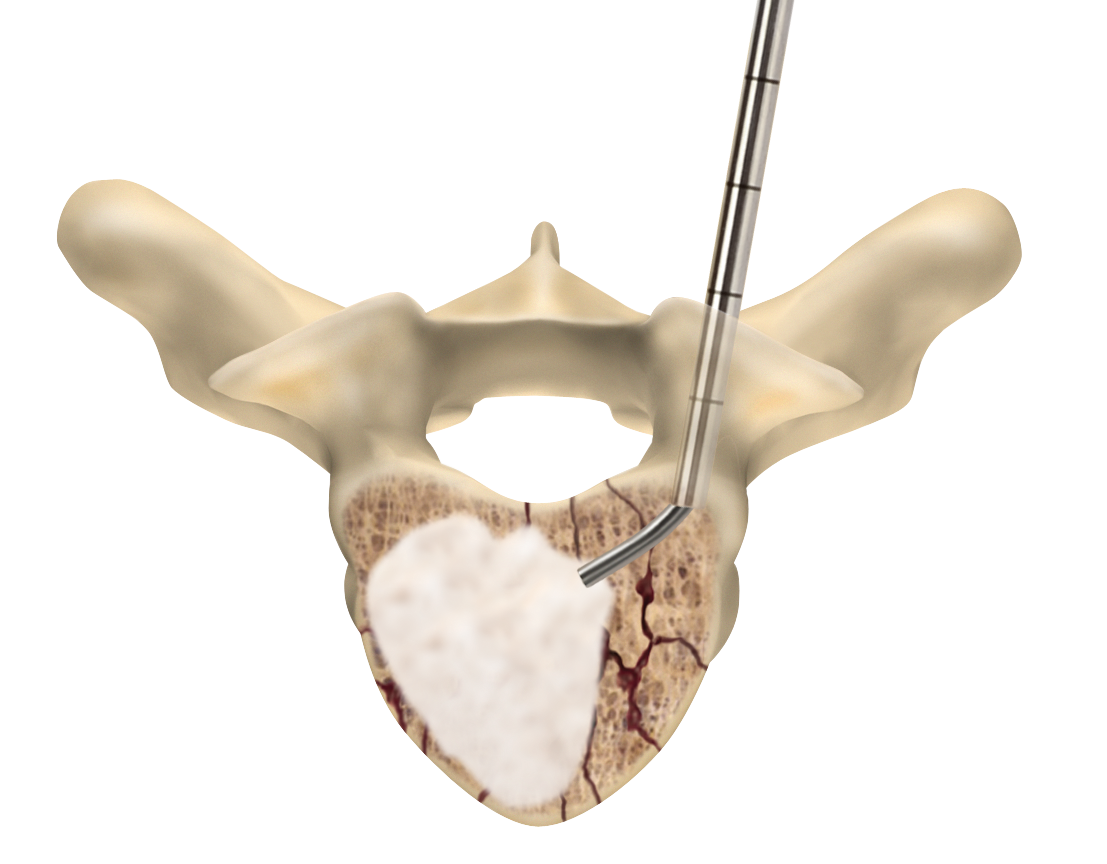
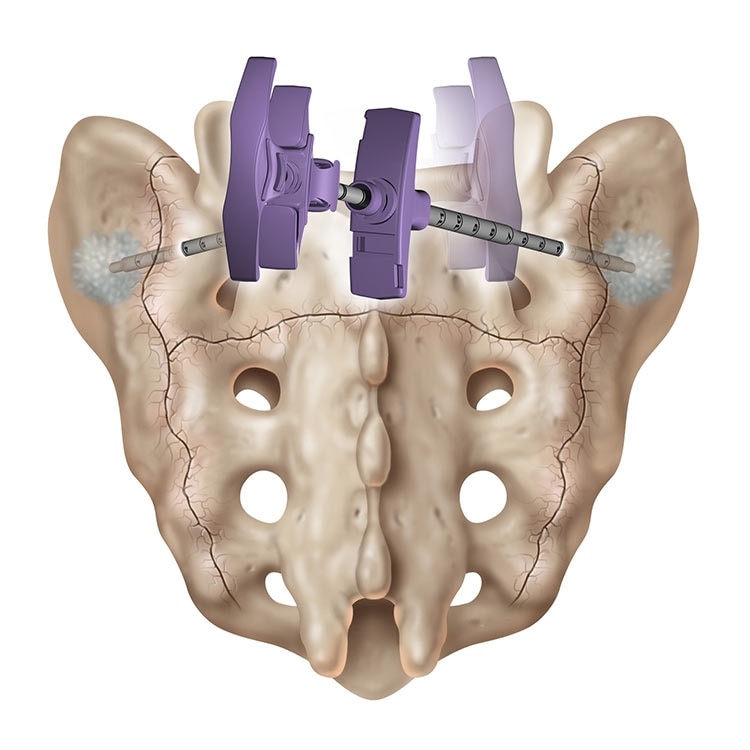
Sacroplasty
A variation of vertebroplasty, sacroplasty involves injecting bone cement into the sacrum to treat sacral insufficiency (SI) fractures.
1. Medtronic Data on File. Business Insights and Analytics, Sept 2020.
2. Boonen S, et al. Balloon kyphoplasty for the treatment of acute vertebral compression fractures: 2-year results from a randomized trial. J Bone Miner Res. 2011;26(7):1627–1637.
3. Van Meirhaeghe J, et al. A randomized trial of balloon kyphoplasty and nonsurgical management for treating acute vertebral compression fractures (vertebral body kyphosis correction and surgical parameters). Spine. 2013;38(12):971–983.
4. Ni W,et al. Trends in opioid use following balloon kyphoplasty or vertebroplasty for the treatment of vertebral compression fractures. Osteoporos Int. 2022;33(4):821–837.
5. Hirsch J, et al. Management of vertebral fragility fractures: A clinical care pathway developed by a multispecialty panel using the RAND/UCLA Appropriateness Method. Spine. 2018;18(11):2152–2161.
