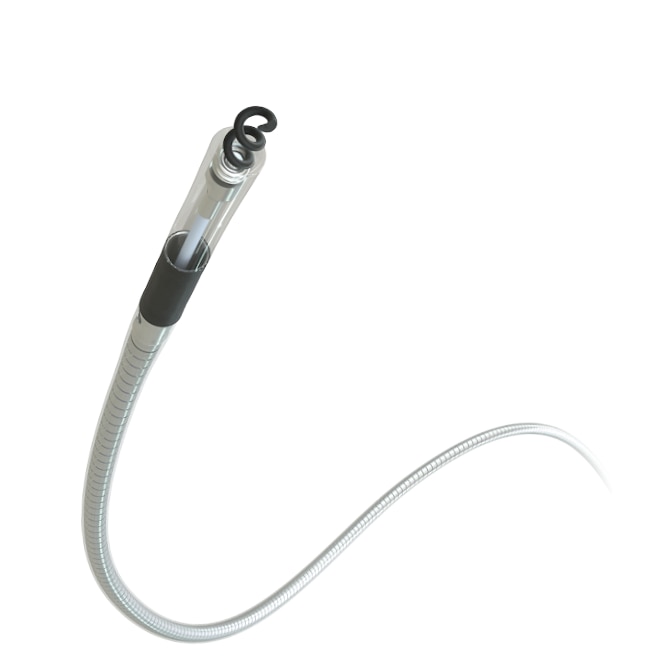
SelectSecure™ MRI SureScan™ Model 3830 pacing lead
for Bradyarrhythmia Management
Model 3830 is the first MR Conditional-approved pacing lead for conduction system pacing, to help treat bradycardia patients who have a pacemaker.
Overview
Approved for left bundle branch area and His-bundle pacing
The 3830 lead is the first FDA-approved lead for providing conduction system pacing, including both His-bundle pacing (HBP) and left bundle branch area pacing (LBBAP).
Conduction system pacing (CSP) is approved to provide physicians an alternative to right ventricular pacing in a single or dual chamber pacing system in bradycardia patients.
Product details
First and only FDA and CDSCO approved
The first and only FDA and CDSCO approved MRI Surescan approved for left bundle branch area pacing, when paired with Medtronic MRI SureScan systems.
Proven safe
3830 use in the left bundle branch area is supported by real-world evidence encompassing 20k+ patients.1

Proven design
3830 has a novel lumenless design and fixed helix, allowing for increased helix stability at implant relative to extendable/retractable helix leads.1,2
Proven reliable
The fatigue performance of the 3830 lead in a left bundle branch area position compares to performance in the RV (98% reliability, 95% CI) based on simulated 10-year testing.3
References
SelectSecure 3830 Left Bundle Branch Area Pacing Safety and Efficacy Utilizing RWE. Medtronic data on file.
Riley WF, Zachary LW. Introduction to Mechanics of Materials. Hoboken, NJ: Wiley; 1989.
Zou J, Chen K, Liu X, et al. Clinical use conditions of lead deployment and simulated lead fracture rate in left bundle branch area pacing. J Cardiovasc Electrophysiol. Published online February 4, 2023.
Response Care: 1-800-419-6700 8:00 am to 11:00 pm, Monday – Saturday (Except Public Holidays)
Email: rs.indiaresponsecare@medtronic.com