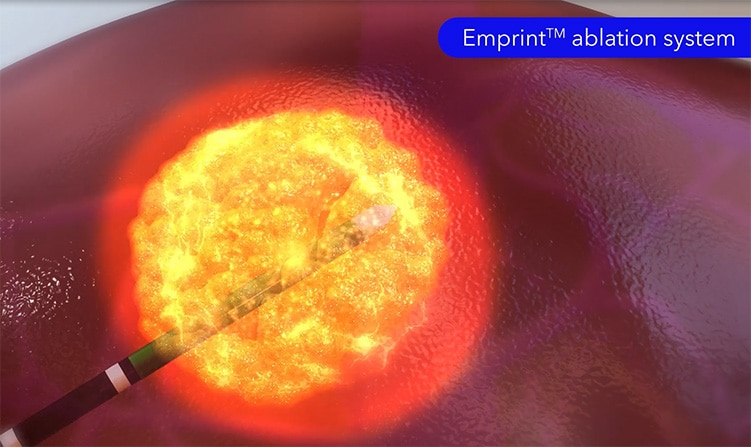
Emprint™ ablation system with Thermosphere™ technology
The Emprint™ ablation system enables a minimally invasive procedure with recovery time,11,12 high accuracy,7 and predictable margins.7
Emprint™ percutaneous antennas with Thermosphere™ technology
The Emprint™ ablation generator is compatible with the Emprint™ percutaneous ablation antenna. Our patented Thermosphere™ technology creates a predictable ablation zone focused at the end of the antenna, with no significant influence from varying tissue conditions.7
Antennas are available in three lengths: short (15 cm), standard (20 cm) and long (30 cm).