ClosureFast™ Radiofrequency Ablation (RFA) System
for chronic venous Insufficiency (CVI)
The ClosureFast™ procedure uses radiofrequency energy to treat patients with superficial vein disease. Its segmental approach features an overlap of 0.5 cm at each treatment segment, designed to eliminate gaps in treatment.
Overview
Data are from ClosureFast 7F studies. ClosureFast 6F catheter is designed to provide the same outcomes as established by ClosureFast 7F catheter.
Catheter inserted
into vein

Controlled heat
collapses vein

Catheter withdrawn,
closing vein

Product details
Proven procedure, lower profile
ClosureFast catheter is now available in 6F, designed for†‡:
- Enhanced flexibility
- Improved navigation
- Increased kink resistance
Deliver lasting results with gap-free thermal ablation
The ClosureFast procedure features a patented overlap of 0.5 cm at each thermal treatment segment, eliminating gaps between segments ― for lasting vein closure.1,3
6F profile, 8 cm heating length

- 8 cm ablation
- 0.5 cm overlap
- 8 cm ablation
Note: 3 cm (7F) heating length available for shorter segments.
Clinical evidence
91.9%
closure at 5 years1
94.9%
reflux-free rate at
5 years1
72%
improvement in VCSS scores at 5 years4
2.5 M+
patients treated5
200+
published articles and clinical studies6
2006
U.S. FDA clearance
7F catheter
Data are from ClosureFast 7F studies. ClosureFast 6F catheter is designed to provide the same outcomes as established by ClosureFast 7F catheter.
Indications
The ClosureFast endovenous radiofrequency ablation (RFA) catheter is intended for endovascular coagulation of blood vessels in patients with superficial vein reflux.
System components
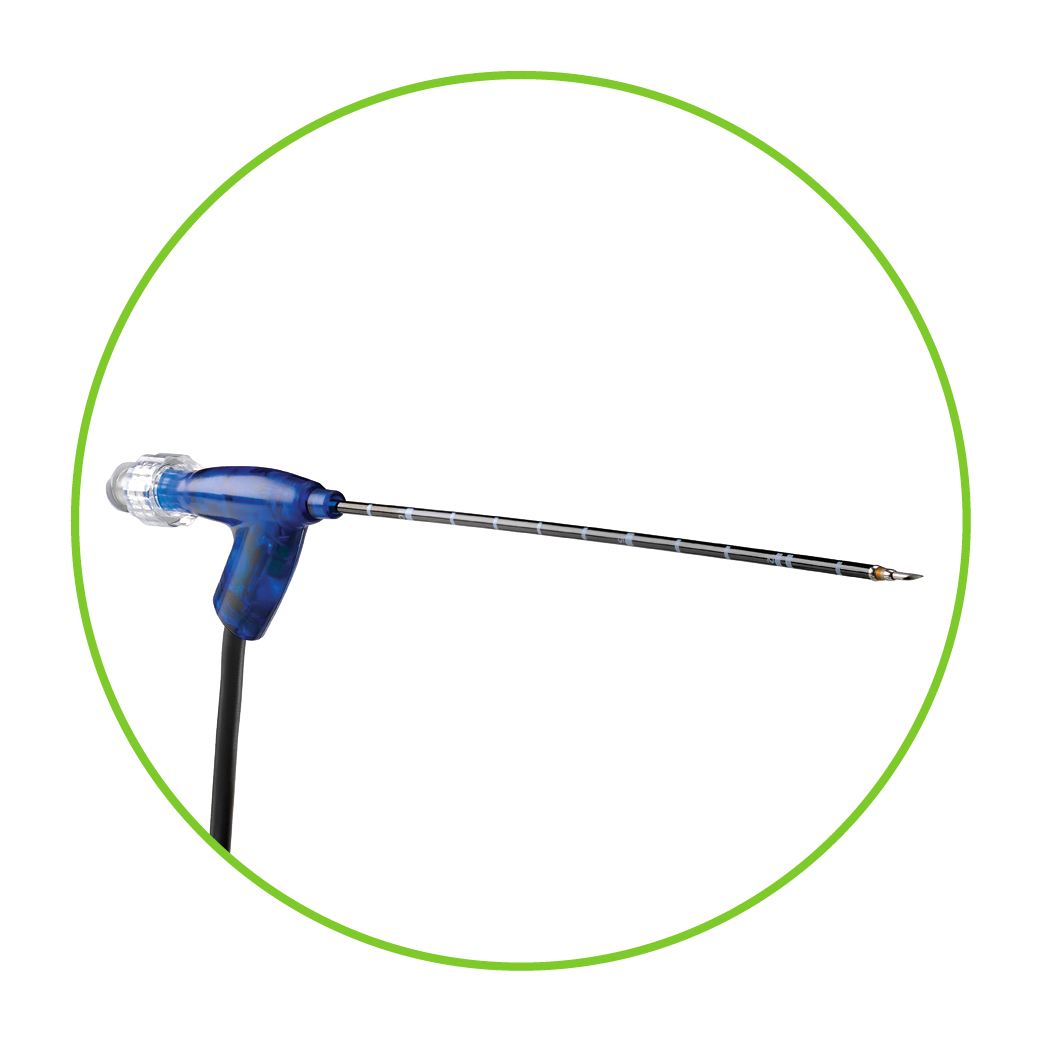
ClosureFast endovenous radiofrequency ablation catheter
- The ClosureFast catheter precisely heats an 8 cm vein segment (or 3 cm for shorter, refluxing vein lengths) in one 20-second interval.
- The heat provided by the catheter shrinks and collapses the target vein, creating a fibrotic seal and occluding the vessel.

ClosureFast endovenous radiofrequency ablation stylet
- The ClosureRFS™ stylet is an intravascular ablation device specifically intended for the treatment of incompetent perforator and tributary veins.
- This minimally invasive outpatient procedure leaves minimal scarring at the puncture site and can be either the primary treatment or an adjunct treatment using the ClosureFast catheter.
ClosureFast radiofrequency generator
- The ClosureRFG generator delivers radiofrequency energy to the ClosureFast catheter and the ClosureRFS stylet for the effective treatment of CVI.
- The controlled feedback mechanism monitors intravascular heat parameters in real time to automatically regulate therapeutic power.

Adverse events may include hematoma, phlebitis, skin injury, nerve injury, thrombophlebitis, thrombosis, and/or pulmonary embolism.
Model specifications
| Catheter model |
CF6-8-60 |
CF6-8-100 |
CF7-7-60 |
CF7-7-100 |
CF7-3-60 |
|---|---|---|---|---|---|
| Introducer sheath (min. ID size) |
6F (2.0 mm) |
6F (2.0 mm) |
7F (2.3 mm) |
7F (2.3 mm) |
7F (2.3 mm) |
| Insertable length |
60 cm |
100 cm |
60 cm |
100 cm |
60 cm |
| Heating element diameter |
2.0 mm |
2.0 mm |
2.3 mm |
2.3 mm |
2.3 mm |
| Heating element length |
8 cm |
8 cm |
7 cm |
7 cm |
3 cm |
| Max. power setting |
40 W |
40 W |
40 W |
40 W |
18 W |
| Default target temp. setting |
120 C |
120 C |
120 C |
120 C |
120 C |
| Software version: RFG2 |
4.0.0 or higher |
4.0.0 or higher |
4.0.0 or higher |
4.0.0 or higher |
4.4.0 or higher |
| Software version: RFG3 |
1.11.0 or higher |
1.11.0 or higher |
1.11.0 or higher |
1.11.0 or higher |
1.11.0 or higher |
| Compatible guidewire |
0.025 in |
0.025 in |
0.025 in |
0.025 in |
0.025 in |
Related pages
Connect with us
Connect with a Medtronic representative and receive updates and ongoing information.
VenaSeal closure system
™*Third-party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company.
Compared to ClosureFast 7F catheter and Venclose™* catheter in bench testing.
Bench test data on file at Medtronic. N = 15 Venclose™* catheters and 30 each ClosureFast 6F and 7F catheters. Bench tests may not be indicative of clinical performance.
References
Proebstle TM, Alm BJ, Göckeritz O, et al. Five-year results from the prospective European multicentre cohort study on radiofrequency segmental thermal ablation for incompetent great saphenous veins. Br J Surg. February 2015;102(3):212–218.
Almeida JI, Kaufman J, Göckeritz O, et al. Radiofrequency endovenous ClosureFAST versus laser ablation for the treatment of great saphenous reflux: A multicenter, single-blinded, randomized study (RECOVERY Study). J Vasc Interv Radiol. June 2009;20(6):752–759.
ClosureFast and ClosureFast RFS Patents. Medtronic data on file, 2022.
Morrison N, Gibson K, Vasquez M, Weiss R, Jones A. Five-year extension study of patients from a randomized clinical trial (VeClose) comparing cyanoacrylate closure versus radiofrequency ablation for the treatment of incompetent great saphenous veins. J Vasc Surg Venous Lymphat Disord. November 2020;8(6):978–989.
ClosureFast Procedures. Medtronic data on file, 2022.
Medtronic ClosureFast Procedure Publications. Medtronic data on file, 2022.
